Soil can either be acidic or alkaline depending on the parent material it was made from. The chemical composition affects the acidity or alkalinity of a soil, a factor which influences the availability of nutrients to plants. Soil acidity is also affected by the use of both natural and chemical fertilisers, lime and leaching.
SOIL pH REACTION
Soil pH is a measure of the acidity vs the alkalinity of the soil, and determines the capacity of that soil to exchange nutrients with plants growing in it. The soil acidity or alkalinity is influenced by the existence of hydrogen ions (H+) and hydroxyl ions (-OH). Therefore, soil pH is the degree of acidity or alkalinity of a soil.
If soil contains more hydrogen ions, it is said to be acidic. If more hydroxyl ions than hydrogen ions are present, the soil is alkaline. A soil with equal members of hydrogen ions and hydroxyl ions is neutral. Soil acidity or alkalinity is measured on a pH scale. The pH scale consists of a series of a number from 1 to 14.
As well as affecting the ability of plants to uptake nutrient by both chemical and biological processes, the pH also affects the diversity and species of soil microbiology. pH is usually measured on a scale of 1-14:
- A pH of 7 indicates neutral soil
- A pH above 7 indicates alkaline soil
- A pH below 7 indicates acidic soil
Soils with less than pH 7 are acidic and those with more than pH 7 are alkaline. This is as shown below
The degree of acidity or alkalinity on a pH scale is either 10 times more or less from one number to the next, depending on the direction on the pH scale.
1. Problems with high pH (alkaline) soils
Soil alkalinity is mainly caused by bicarbonates and carbonates, although phosphates, borates and some organic molecules can contribute. In a soil with pH from 7 to 8.2, bicarbonates and carbonates of calcium and magnesium dominate.
Calcareous soils contain from 1 to 90 % lime material as calcium carbonates and these sparingly soluble salts cause the soil to have a pH of 8.0–8.2 which is not a severe problem for plant growth or agricultural production.
Problems are encountered in alkaline soils when sodium occurs or accumulates and forms salts such as sodium bicarbonate and sodium carbonate. These are highly soluble and increase the soil pH above 8.
When the pH is more than 9, the soils are considered highly alkaline and often have toxic amounts of bicarbonate, carbonate, aluminium and iron. Nutrient deficiency is also likely to be a major problem and the high amount of exchangeable sodium in these soils reduces soil physical fertility.
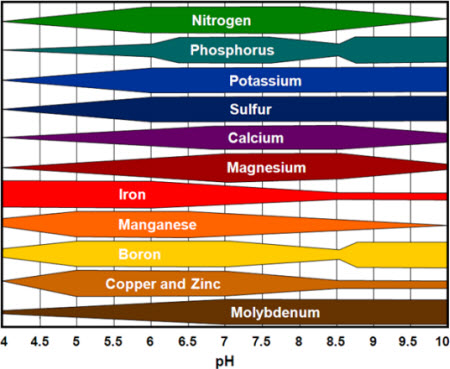
Most nutrients that plants need can be chemically assimilated when the pH of the soil solution ranges from 6.0 to 7.5.
- Below pH 6.0, some nutrients, such as nitrogen, phosphorus, and potassium, are less available.
- When pH exceeds 7.5, iron, manganese, and phosphorus are less available.
So getting your soil pH right is absolutely essential, if you want nutrient-dense vegetables.
Not all plants are the same – different crops prefer different levels of acidity – for example:
- Strawberries will yield well at a lower pH of 5.5 to 6.5
- Carrots love balanced soils pH of 6 to 7
- Sunflowers thrive in soils ph 7 to 7.5
So it depends on what you are growing as to what pH you want to nudge your soils towards. Keep in mind that most annual veggies prefer a bacterially dominated soil, which is leaning towards a pH of 7 – 7.5
Experiment: Testing the soil with a simple soil ph test kit:
Requirements: a soil pH test kit including the following:
We recommend the dye & powder system or Kelway Soil pH & Moisture Meter for broader areas.
- Use a small sample of soil, taken 10-15cm from the surface, and put on the mixing card
- Add a few drops of the indicator dye and dust with the white powder supplied with the kit.
- Wait about 30 seconds for the colour change to take effect. You will get a more accurate result if you wait a few minutes.
- Use the colour chart to match the colour of your soil samples. Each colour indicates what level pH your soil is.
- If in doubt, wait 2 minutes and check again the resulting colour
- Take several measurements in different spots in the garden. A minimum of six samples from different parts of the garden is a safe amount.
- A single reading may be an anomaly, so it is good to get an idea of the average pH in a plot. If they are all around the same, take the average and amend the soil accordingly. If one spot is very different than the rest, however, there is need to “spot treat” it.
- Record your results. You may need to reference your test results at a later date, as they may change over time.
- Test your soil annually to know exactly what your garden’s nutritional requirements are.
It is important to note that keeping a garden diary is a great way to improve garden and soil health for it is difficult to remember all the pH results just in one’s head
2. How to balance your soil’s pH
Once the soil is tested in at least six places and is found to be generally a pH of different from 7, the following measures should be taken:
If the soil is too acidic: less than 7 = low pH:
- Add green manure crops into your rotation with more frequency.
- Add organic matter in the form of a well-balanced, pH neutral compost, adding humus is the best way of changing pH.
- Add agricultural lime (not builders lime). As a rule of thumb, carefully apply 100g to each square metre. NOTE lime can only be accurately applied if a total mineral test is performed. It will take a while to increase the pH this way , so you should see a change in the pH within 6 months. Be careful not to over apply.
- Add Dolomite , though it contains Magnesium, which if it is already present in large quantities, could block other minerals. Again, a total mineral test is a good idea before doing this.
If soil is too alkaline: greater than 7 = high pH:
- This soil will be harder to rebalance
- Add organic matter such as pine needles or decomposed tree leaves.
- Add green manure crops into your rotation with more frequency
- Add organic matter in the form of a well-balanced, pH neutral compost, adding humus is the best way of changing pH.
- In an extreme situation you could use powdered sulphur. Be very careful with this as sulphur is anti-microbial and will kill off the important microorganisms in the soil if applied regularly. Apply one handful per square metre, once a year. It works very slowly and the change in the pH may not easily noticed before about 6 months.
3. Significance of soil pH for plant growth
Some of the main effects of pH on plant growth are as follows:
Low pH lowers the availability of nutrients such as phosphorus and molybdenum. On the other hand, at alkaline pH above 8.5,” manganese, potassium, boron, iron and zinc become less available.
At very low pHs the concentration of available iron and aluminium in the soil solution may increase to such an extent as to become injurious or toxic to the plants.
Very acidic or low pHs inhibits the activity of soil microorganisms, notably the nitrifying and nitrogen-fixing bacteria.
Soil pH may affect the balance of the different microorganisms in the soil by influencing their competitive ability.
Plant damage by various soil pests e.g. nematodes is usually more serious in acid than in neutral soils. Different crop species react differently to pH.